 2025-07-23
2025-07-23
Aseptic sampling valves are installed on the side of the pipeline or the side of the tank. They are used to regularly extract random samples during the production process. They are key components in the pharmaceutical and biotechnology industries. They are used to take samples from process pipelines or containers to avoid introducing contamination. And designed to be easy to clean and sterilize, and use a closed system to minimize the risk of contamination during sampling. Key aspects include understanding pre-operation preparation, working principles, connection types, sterilization methods, and precautions required for safe and reliable operation.
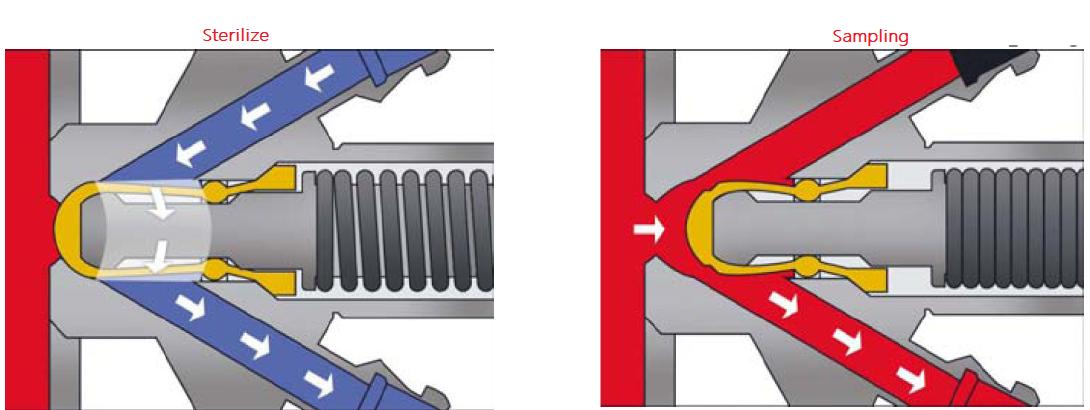
The sampling valve is mainly composed of a valve body, a sealing ring, and an actuator. The valve stem is controlled by the actuator to move axially up and down, and the sealing ring is connected to the valve stem.
When the valve stem moves upward, the sampling valve opens and the sampling operation is performed; when the valve stem moves downward, the sealing ring is reset, the sampling valve is closed, and the sampling ports on both sides can be CIP cleaned and SIP sterilized.
Ensure that the sampling area is a clean environment and check whether the environmental test data meets the standards.
Ensure that the sampling valve is sterilized. Use saturated steam for sterilization. The saturated steam pressure is required to be ≤1bar(g).
The aseptic sampling valve should ensure that the center line of the sampling valve is at a horizontal angle, and the sampling port is installed vertically to ensure that the valve can be self-drained.
Sterilization before and after sampling is essential. Steam sterilization in place (SIP) and cleaning in place (CIP) methods ensure that the valve and its connected equipment reach the required sterilization temperature (for example, steam sterilization temperature is 121°C).
Valves have specific temperature and pressure ratings that must be strictly followed. Exceeding these limits may compromise the integrity of the valve and cause failure.
The material of the diaphragm or seal is essential to maintain sterility. Please ensure that it is compatible with the product and the sterilization process. After reaching the specified number of uses (for example, after 100 samplings or sterilization), please replace the diaphragm.
Sterilize the valve
Clean or sterilize the valve.
Open the sampling valve and perform sampling operations
After sampling, close the sampling valve.
Clean or sterilize the valve
Empty the medium in the valve and keep it dry, and store it in a dry environment.
The storage temperature should not be too high or too low, and the suitable storage temperature is 20℃-25℃ (normal temperature).
The storage environment requires ventilation and dust-free.
After storage, before reactivation, please check whether the valve opens normally to ensure that there is no jamming.
Using irritating cleaning products such as corrosive soda and nitric acid may cause skin burns. Wear rubber gloves and goggles when cleaning.
Regular maintenance, including cleaning and inspection, is essential for reliable operation. Replace worn or damaged parts in a timely manner.
1. Aseptic control: Avoid prolonged exposure of the valve to a non-sterile environment when the valve is open. Avoid touching the valve joint or the inside of the sampling container with bare hands.
2. Valve maintenance: Regularly check whether the diaphragm and sealing ring are aging to prevent leakage or contamination.
3. Cross-contamination risk: Sterile tools need to be replaced or thoroughly sterilized between different batches of samples.
4. Safety matters: Before using the valve, please read the manufacturer's operating manual in detail. Work related to the valve requires experienced personnel to operate.
Why sanitary stainless steel butterfly valves corrosion resistant?